SCD News Hub
Challenges and opportunities in delivering gene therapies for sickle cell disease and beta thalassemia
The first study assessing the real-world commercial roll-out of gene therapies for sickle cell disease and beta thalassemia offers lessons learned to inform best practices as manufacturers and medical centers prepare to meet growing demand for gene therapies in the coming years.
Read moreNew evidence shows hematopoietic cell transplantation offers durable relief for sickle cell disease

Patients who underwent hematopoietic cell transplantation for sickle cell disease saw high rates of survival without disease symptoms and low rates of severe side effects or complications years after their procedure, according to a new study.
Read moreFulcrum Therapeutics Announces Positive Initial Results from the 20 mg Dose Cohort of the Phase 1b PIONEER Trial of Pociredir in Sickle Cell Disease at the 67th American Society of Hematology Annual M

― Clear dose-response observed, with a robust and clinically meaningful fetal hemoglobin induction at the Week 6 timepoint : mean absolute HbF in the 20 mg cohort increased by 9.9% at Week 6 ; 7 of...
Read moreMost sickle cell patients face long delays for recommended pain relief, study shows

A new study finds that only one in three patients visiting emergency departments (EDs) for severe pain associated with sickle cell disease received appropriate opioid-based pain-relieving medications within the first hour as recommended by the American Society of Hematology (ASH) and National Heart, Lung, and Blood Institute (NHLBI).
Read moreExa-cel shows complete success in early pediatric trials for sickle cell disease and beta-thalassemia
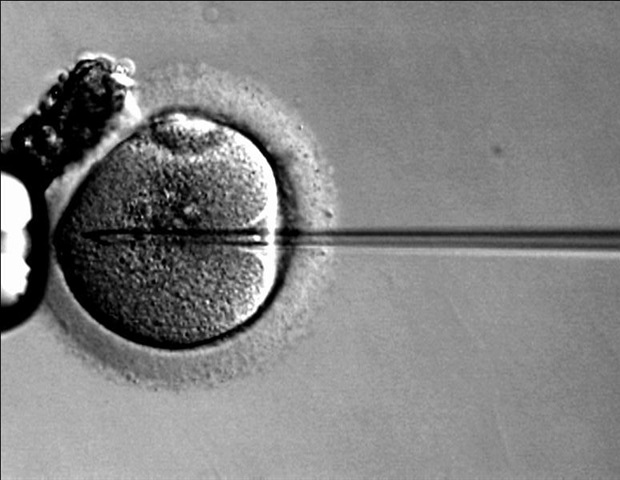
Preliminary results from two trials of the gene therapy exagamglogene autotemcel (exa-cel) suggest the therapy offers an effective cure for beta-thalassemia and sickle cell disease in children younger than 12.
Read moreVertex Pharmaceuticals Incorporated Presents New Data on CASGEVY, Including First-Ever Data in Children Ages 5-11 Years, at the American Society of Hematology Annual Meeting and Announces Plan for Glo

Vertex Pharmaceuticals Incorporated announced data from multiple studies demonstrating the clinical benefits of CASGEVY®? in people ages 5-11 years and older living with severe sickle cell disease ...
Read moreBeam Therapeutics Reports Updated Data from BEACON Phase 1/2 Trial of ristoglogene autogetemcel (risto-cel) Highlighting Durable, Differentiated Profile in Sickle Cell Disease (SCD) at American Societ

Updated Data from 31 Adult and Adolescent SCD Patients Treated with risto-cel (Formerly BEAM-101) Show Mean Hemoglobin F (HbF) Induction of >60%, Hemoglobin S (HbS) Reduction to Resolution of Anemia Durable for up to 20 Months
Read moreVertex Presents New Data on CASGEVY®, Including First-Ever Data in Children Ages 5-11 Years, at the American Society of Hematology Annual Meeting and Announces Plan for Global Regulatory Submissions

Data from pivotal studies of CASGEVY in children ages 5-11 years with severe sickle cell disease or transfusion-dependent beta thalassemia demonstrates the transformative potential of the therapy in...
Read moreVertex's gene therapy shows promise in younger children with blood disorders

Vertex Pharmaceuticals said on Saturday its gene therapy helped children aged between 5 and 11 years with sickle cell disease to be free of painful events and allowed those with another blood disorder that requires frequent blood transfusions to be transfusion-free for at least 12 consecutive months.
Read moreVertex's gene therapy shows promise in younger children with blood disorders

Vertex Pharmaceuticals said on Saturday its gene therapy helped children aged between 5 and 11 years with sickle cell disease to be free of painful events and allowed those with another blood disorder...
Read more
