Gene Therapy in Sickle Cell
For nearly 100 years, scientists have studied the human body and the building blocks of DNA. With each mark of progress comes a better understanding of how our bodies work at the genetic level. We are constantly learning about how changes to our DNA can prevent our genes from working correctly, sometimes leading to diseases such as sickle cell disease.
Sickle cell disease is a lifelong, genetic illness caused by a mutation in a gene at the DNA level. The mutation affects the normal adult hemoglobin (HbA) gene, leading the body to produce an abnormal form of hemoglobin called sickle hemoglobin (HbS). This can cause red blood cells to form in the shape of a sickle.
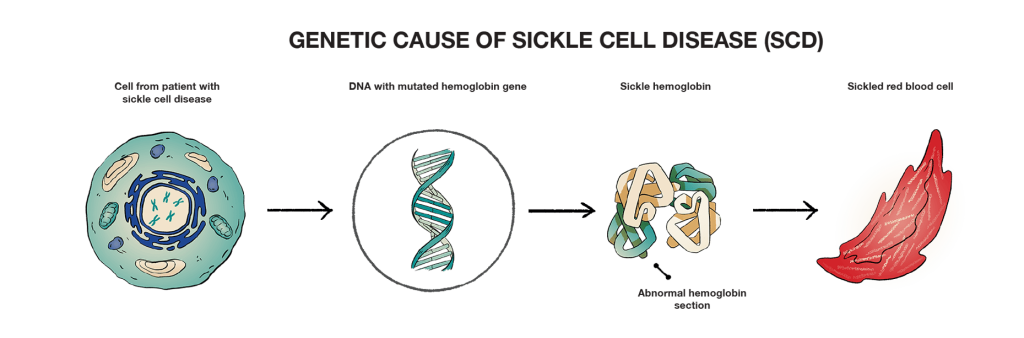
The more research and understanding we gain, the better scientists can determine how to target these changes to treat diseases at the source—at the genetic level.
Today, there are multiple gene therapies that are being researched and investigated as possible treatment options for sickle cell disease—all built on the strong foundation of decades of DNA research.
What Is Gene Therapy?
GENE THERAPY RESEARCH
Though gene therapy has long been studied as a potential treatment option, it has made progress toward treating inherited diseases and some cancers. A few gene therapies have already been approved by the U.S. Food and Drug Administration in therapeutic areas other than SCD, like some rare genetic diseases and cancers. Gene therapy for sickle cell disease is currently not FDA-approved. Safety and efficacy have not been established.
TYPES OF GENE THERAPY
Currently, the most advanced clinical studies in sickle cell disease are focused on 2 types of gene therapy: gene addition and gene editing. Both use a patient’s own stem cells for treatment so patients do not need a donor. There are other types of gene therapy outside of gene addition and gene editing that are in preclinical research and early clinical development for sickle cell disease as well.
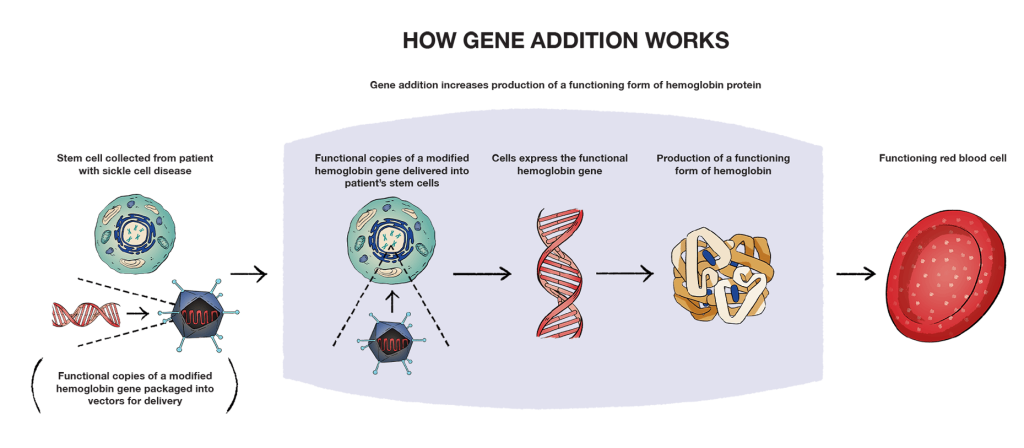
Gene addition works by adding modified, working copies of a gene to stem cells collected from a person with sickle cell disease to take over the function of a missing or abnormal gene. So, for patients with sickle cell disease, that would be adding a working gene that instructs the body to produce a functioning form of hemoglobin that can compensate for sickle hemoglobin (HbS). In gene addition, the genetic material needs a way to get inside the cells of the person getting treatment. This happens through a delivery system called a vector.
The job of a vector is to deliver the functional copies of a modified hemoglobin gene to the cells so that the body can begin to make a functioning form of hemoglobin. Vectors are well studied and ideal delivery systems for delivering genetic material into a person’s cells. One of the most widely used and studied vector types are lentiviral vectors (LVVs). The reason scientists create vectors based on viruses is because viruses are very efficient at delivering genetic material to cells of the body. Though vectors are based on viruses, they do not contain any of the parts of the virus that can cause an infection. They act as a delivery system for cells.
Gene Editing
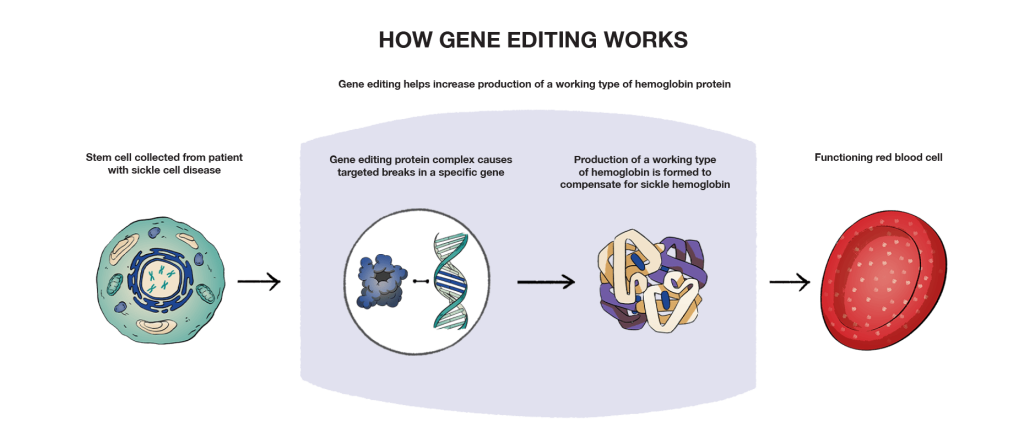
Gene editing modifies a person’s DNA by changing either the mutated gene directly, or by changing a related gene to compensate for the mutated one. In current sickle cell disease research, this means turning the on/off switch for a different gene that regulates the production of a type of hemoglobin produced by your body. This switch helps the body increase production of a working type of hemoglobin protein.
One of the latest types of gene editing technology being explored is known as CRISPR-Cas9, or the Clustered Regularly Interspaced Short Palindromic Repeats found in bacteria.
Similar to gene therapy, gene editing requires the collection of stem cells from a patient to start the process. Gene editing is used within stem cells to help create changes in the DNA of a gene.
In addition to gene editing and gene addition, there are other types of gene therapy being studied as part of the greater sickle cell disease treatment landscape, like gene correction and gene silencing. These different types of gene therapy continue to evolve rapidly, with many current and upcoming clinical trials underway to investigate their potential as treatment options for sickle cell disease.
Gene Therapy Treatment Process
The treatment process for gene therapy relies on close collaboration between a patient and their primary physician, their treatment team, and their caregivers. It’s important to keep in mind that there are a lot of things to consider when learning about gene therapy. It can be a detailed process—one that takes place over a long period of time, often with many consultations and visits. That’s why staying informed and having as many conversations as needed are important aspects of the process.
Since gene therapy in sickle cell disease is not FDA-approved, the only way to receive treatment is to participate in a clinical trial, where patients must meet strict eligibility criteria and be informed about the potential risks and benefits of participating in the trial. Learn more about clinical trials here.
In general, there are 4 steps to the treatment process: consultation, preparation, treatment, and recovery. Let’s take a quick glance at each step:

STEP 1: CONSULTATION
- Discuss the potential risks and benefits of the specific gene therapy
- Ensure appropriateness and eligibility of gene therapy. This can include discussions around your or your loved one’s physical and emotional health, as well as their existing support network
- If you or your loved one decides to discuss or move forward with gene therapy, you or your loved one will need to go through a process called informed consent. Informed consent is where a patient actively participates in discussion with their physician and is empowered to make decisions about their medical care by deciding which treatments they do (or do not) want
- Discuss any short- and long-term steps to plan for gene therapy, including healthcare coverage, fertility discussions, timing of treatment, chemotherapy, side effects of gene therapy, and any potential impacts on life, family, and work
- This consultation step can take weeks or months, depending on discussions between you or your loved one and the treating physician/treatment team and timing of referral. These discussions can include multiple consultations with specialists/physicians at a specialized treatment center with expertise on gene therapy
- You or your loved ones’ primary physician and care team at the treatment center will work with you to collectively determine if gene therapy is the appropriate treatment choice

STEP 2: PREPARATION
- Will include the stem cell collection needed for manufacturing gene therapy ex vivo
- Physician will give instructions for any preparation procedures or regimens that your or your loved one will need to complete
- Usually requires a few days for the patient to stay in the hospital for stem cell collection. After the collection, it will usually take a few months for the lab to modify the patient’s own stem cells to create the gene therapy

STEP 3: TREATMENT
- Conditioning with chemotherapy happens at this stage—conditioning is needed for all transplant-based treatments to help maximize the effects of treatment by clearing out the cells that contain the mutated hemoglobin gene and making room in the bone marrow for the modified stem cells
- Gene therapy treatment that was prepared using your or your loved one’s own cells is infused back in. Treatment is often administered by a specialist and can include a specialized care team
- After treatment, you or your loved one will remain in the hospital for several weeks—this time span includes a recovery period and the time needed for engraftment and monitoring. Engraftment is when transplanted stem cells enter the blood and make their way to the bone marrow to start making new blood cells

STEP 4: RECOVERY/FOLLOW-UP
- You will be discharged once your physician has decided it is safe for you to leave the hospital
- May include follow-up appointments at the specialized treatment center and with you or your loved one’s physician
- May include home healthcare for a period of time
- May include enrolling in a registry to monitor long-term treatment results
After gene therapy is administered, there is a critical process of recovery and a follow-up period that can last for a number of years. If you or your loved ones are treated with gene therapy, you will work with your physicians to monitor the effect of treatment for the long term, which may be several years. As gene therapies continue to be studied for different genetic diseases, you or your loved ones may be asked for your interest in enrolling in registries to help track the long-term outcomes of treatment.
Potential Risks of Gene Therapy
As with any treatment, there are risks associated with gene therapy. The risks depend on the type of gene therapy, type of vector (used to deliver the gene therapy), and the administration method. Some risks can be serious. The safety of gene therapy will continue to be assessed over time.
Some potential risks of gene therapy include:
- Insertional Oncogenesis: In gene addition, the new DNA (genes) from the vector insert into the DNA of the person’s stem cell amongst their other genes. This process is called integration. Most integration causes no harm to the cells or the patient. However, there is a chance that the integration of the new DNA may change the activity of nearby genes. If one of these genes controls cell growth, the integration of the vector may cause uncontrolled growth of the cell, after it is returned to the person, resulting in cancer. Insertional oncogenesis is the term used for a cancer that has been caused by the new DNA inserting in an unwanted location.
- Off-Targeting: In gene editing, there is a possibility that the technique used could make changes at a different site than the one intended. This can cause changes to healthy genes, which may have unintended effects.
- Unintentional Gene Inactivation: With any type of gene therapy, there is the risk of unexpected complications that unintentionally prevent the function of another important gene.
If you have questions, consider a discussion with a US Healthcare provider.
